
Electrolysis of Brine

Figure 1 Diaphragm or Hooker cell for electrolysis I of brine (schematic).

electrolysis of brine

The other product of electrolysis of brine

The electrolysis of molten sodium chloride produces sodium and chlorine.

Electrolysis of salt gives Hydrogen, Chlorine and NaOH

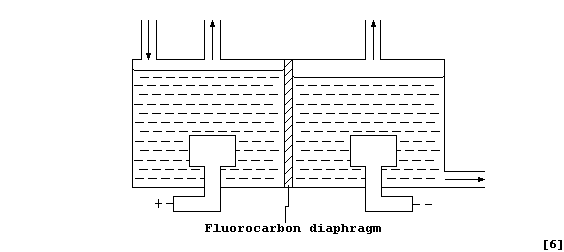
Electrolysis Of Brine: SODIUM HYPOCHLORITE DIAGRAM

If you perform electrolysis on brine you will get three substances: chlorine
![[edit] Electrolysis of Concentrated Sodium Chloride Solution (Brine) [edit] Electrolysis of Concentrated Sodium Chloride Solution (Brine)](http://wiki.one-school.net/images/thumb/d/db/Electrolysis_NaClconcentrated.png/500px-Electrolysis_NaClconcentrated.png)
[edit] Electrolysis of Concentrated Sodium Chloride Solution (Brine)

The processing of brine electrolysis to produce caustic soda, chlorine,

This method is called salt electrolysis. Currently, there are three methods

a partition between the electrodes (Diaphragm) is minimised.
OKK Brine Electrolysis Generator/ WATER IONIZER LP-D2

The electrolysis of aqueous Sodium Chloride (Brine)

Figure 1 An electrolytic cell. The battery pumps electrons away from the

Electrolysis of Brine The solution of sodium chloride in water is known as
electrolytic cell with: Cl2(g); H2(g); NaOH(aq); Saturated brine;

The Nelson diaphragm cell: In the Nelson cell, saturated sodium chloride

Chloralkali electrolysis

A brine solution of NaCl is fed into the anode compartment, where chlorine

Tidak ada komentar:
Posting Komentar